TINY PARTICLES, BIG QUESTIONS: what’s the fuss about titanium dioxide??
‘Natural does not equal safe. There’s a plant in my garden where if you simply sat under it for ten minutes then you’d be dead.’ … I ask him about that plant over a colonoscopy later. ‘Water lily.'
- Adam Kay, This is Going to Hurt: Secret Diaries of a Junior Doctor
HOW DID I GET HERE?
I‘ve known for a while that I’d like to shift my personal-care products to more ‘natural’ options - fewer ingredients, ones that I actually know the origins of.
As I get close to finishing a product, I begin having a look into its ingredients and alternative ‘natural’ options (expect a separate post on some lovely brands I’ve found!).
One substance came up in nearly all of my personal care products:
Titanium Dioxide (TiO₂), often referred to as ‘CI 77891’
Puzzlingly, it was also in nearly all the ‘natural’ options I could find.
I didn’t notice this pattern with any other ingredient, so I began digging further.
TiO₂ is used in cosmetics because it thickens & smooths texture, acts as a whitening agent, increase opacity, and filters UV rays. This all sounds wonderful, until you look at its potential health effects…
MINERAL FORM
Titanium Dioxide occurs in three mineral forms:
Anatase: most commonly used, most reactive to UV light
- photosensitive (releases electrons when exposed to light that then react with other molecules, also known as free radicals)
- often used for photocatalysis (accelerates chemical reactions with absorbed light energy)
Rutile: not particularly reactive to UV light
Brookite: Rarely used, reactive to UV light
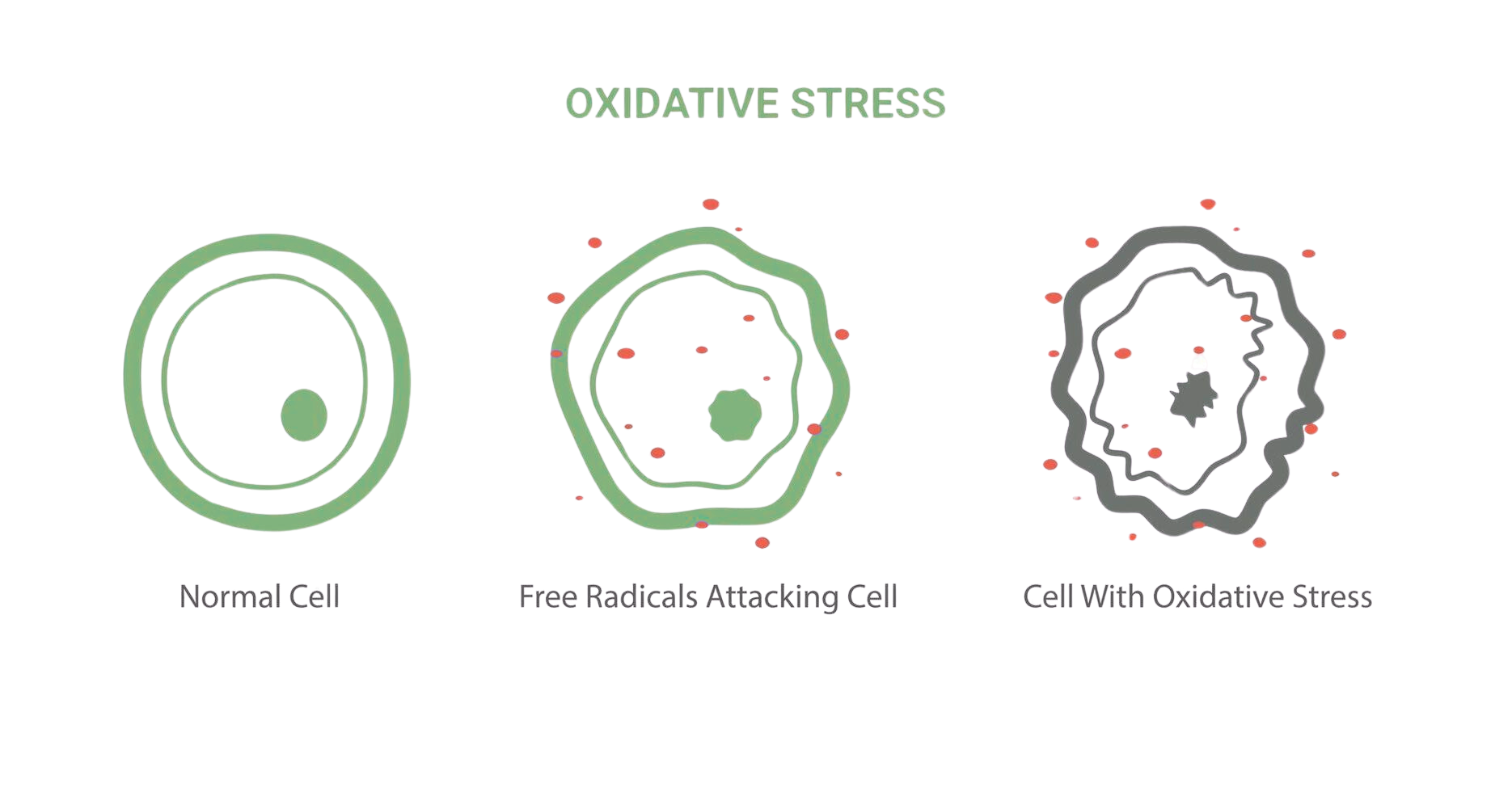
‘Free radicals’ are unpaired electrons seeking to be paired up with other electrons again. To do so they will attack cells to steal electrons from their DNA and protein. Our cells can only cope with a certain amount of this before entering ‘oxidative stress’, becoming overly damaged and potentially leading to serious diseases including cancer. Free radicals are most often released from compounds containing oxygen, like TiO₂.
Beyond health risks, this cellular degradation promotes aging - isn’t skincare supposed to help us stay youthful?!
To limit reactivity some cosmetic companies will add antioxidants to their formulas or coat the TiO₂ with stabilisers like silica, but sadly regulations are lacking in this area.
NANO & NON-NANO
Nano particles, <100 nm
Non-nano particles, >100 nm
Non-nano TiO₂ is used in makeup to provide pigment as the larger particles scatter visible light more effectively and create an opaque pigment. Nano TiO₂ is used in skincare and sun cream because it blends easily into the skin and offers better sun protection, absorbing and reflecting UV rays.
Unfortunately, since nano TiO₂ have a high surface area, they are far more photosensitive and promote more photocatalytic activity. As you know, that’s less than ideal for our skin.
What’s more, once nano particles are washed off down the sink and into our waterways they could be consumed by marine life, devasting our coral and oceans. Moreover, whilst we might not ingest liquid cosmetics initially, we do eat fish…
My research went much further than the above, but I think it’s best to err on the side of caution and keep things concise.
This is a brief summary of my findings.
Ultimately, whether you use products containing titanium dioxide is a personal choice that should involve research beyond this post.
If you have any questions, feel free to email me on rosesandreverieinfo@gmail.com